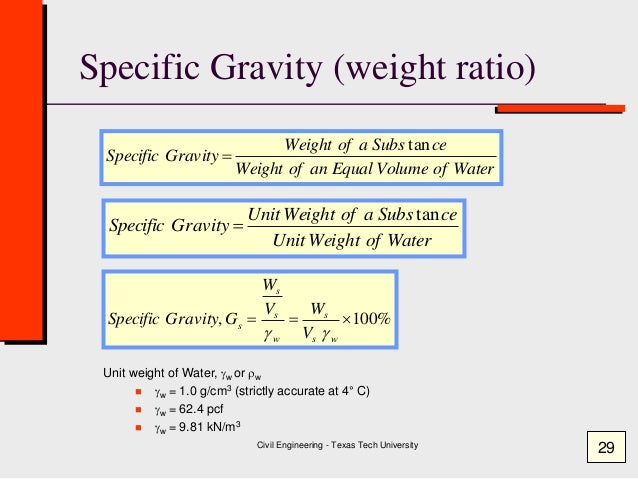
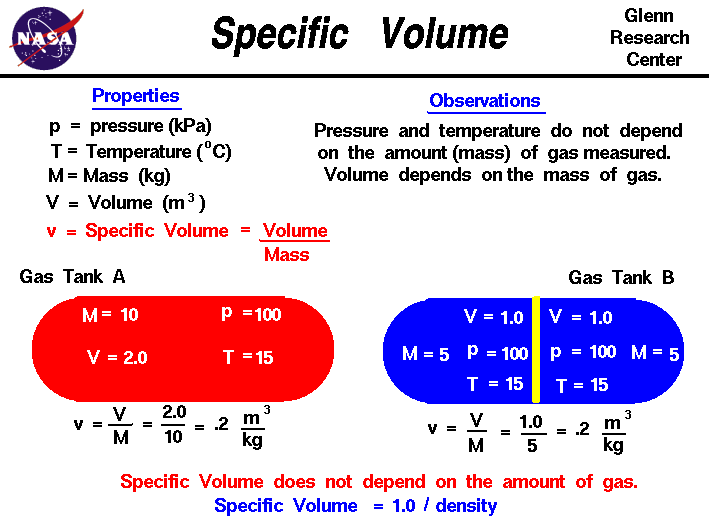
1. Isobaric ( P is constant)
Constant pressure on a T- s
Work done:
W = P(V2 - V1)
or
W= mR(T2-T1)
Unit : kJ
Heat flow:
Q = mCp ln (T2-T1)
Unit : kJ
The change of entropy is
s2 - s1 = mCp ln (T2/T1)
Unit : kJ/K
s2 - s1 = Cp ln (T2/T1)
Unit : kJ/kgK
2. Isometric (V is constant)
Constant volume on a T-s diagram
Heat flow =
Q = mC(T2-T1)
Unit : kJ
Change of entropy:
s2 - s1 = mCv ln (T2/T1)
Unit : kJ/K
s2 - s1 = Cv ln (T2/T1)
Unit : kJ/kgK
3. Isothermal (T is constant)
Constant temperature process on a T-s diagram
(the shaded area represents the heat supplied during the process)
Change of entropy:
s2 - s1 = R ln (v2/v1) = R ln (p1/p2)
Unit : kJ/kgK
s2 - s1 = mR ln (v2/v1) = mR ln (p1/p2)
Unit : kJ/K
4. Adiabatic
Reversible adiabatic process on T-s diagram
W = mCv(T1 - T2)
or since, Cv = R/ γ-1
Or since PV = mRT
W= P1V1 - P2V2/ γ-1
Temperature, Pressure and Volume for perfect gasses:
T2/T1 = (P2/P1)^γ-1/γ = (V1/V2)^γ-1
T2/T1 = (P2/P1)^γ-1/γ = (V1/V2)^γ-1
5. Polytropic Process
Reverse poly tropic process on a T-s diagram
Work done:
W = P1V1 - P2V2/ n-1
or since PV = mRT
W = mR(T1- T2)/ n-1
Change of entropy:
U2 - U1 = mCv(T2-T1)
Heat Flow:
Q = W + U2 - U1
Written by: Cahaya Athirah binti Mohammad Taufik
























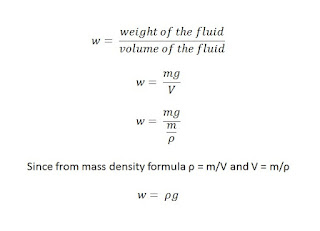 (where g=9.81m/s^2)
(where g=9.81m/s^2)